Email us: contact@thejusticenow.com
Cartiva Implant Lawsuits: Keep the Manufacturers up the Creek - Did you have the knowledge that your toes are twice the weight of your other of your toes? "Big Toe "big" toe is important in moving and absorbing stress. Your big toe should be in good condition and shape when you walk or run. It is responsible for maintaining the equilibrium of your body.
As with every other body part, the large toe can suffer from arthritis. The remedy that doctors have found in the past included toe joint fusion. The procedure makes the joint stiff, resulting in less mobility.
The development of the Cartiva implant for the toe, which made it possible to move the joint easily, gave an excellent resolution. However, patients and clinicians began to notice implant failure and other problems with the toes right after the introduction of the implant. This led to Cartiva Implant lawsuits.
This article outlines the necessity of Cartiva implants. It also discusses Cartiva implant problems and the development and the current state of Cartiva Big Toe Joint Implant lawsuits.
To fully comprehend the importance of an implant for the toe, we must first understand the anatomy of the big toe.
The toes of the big are comprised of two joints, the metatarsophalangeal joint (MTP) and the interphalangeal joint (IP). Metatarsophalangeal Joint (MTP) is the larger of both joints and links the metatarsal (first long bone of the foot) to the phalanx (first bone of the toe).
The area covering MTP and IP joints is known as the plantar complex. It can resist the dorsiflexion of the MTP joint.
Degenerative arthritis in MTP joints creates the platform of the large toe rigid (hallux rigidus), which restricts the range of motion of the big toe (hallux limitus) itchy and painful.
This problem is mostly observed in those between 30 and 60 of age. One in forty individuals is more likely to be affected by large toe arthritis. The surgery to fuse the toes in those with this condition will make running or walking difficult because the metatarsal joint is immovable and lacks flexibility.
Cartiva’s synthetic cartilage implant is a tiny, round gel-like device put in between two edges of the joint to provide a solid and smooth surface that allows the toe big one to walk along with less pain. Polyvinyl alcohol (PVA), along with saline implant material, has properties that are similar to authentic cartilage. It replaces a small amount of the damaged cartilage in the toe joint.
[subscribe_to_unlock_form]In 2015, this revolutionary invention, created by Cartiva, Inc., became a viable alternative to surgery for toe fusion. Its Cartiva(r) SCI device got the Food and Drug Administration‘s 510(K) premarket approval in 2016.
Later, Cartiva was purchased by its main competitor, Wright Medical Group (2018), for a $435 million acquisition.
The year 2020 will see Stryker International, a medical and healthcare products company based out of Michigan, acquire Wright Medical by closing a deal worth $4 billion. Stryker has been manufacturing and promoting products until now.
When Cartiva was approved in the year 2000, it was based upon clinical trials showing a 13.5 percent possibility of failure. But, subsequent studies showed that the failure rate could be higher than 13.5 percent. Some people have heard of over 64% failure rate for curative implants.
In November 2020, research by the American Orthopedic Foot and Ankle Society revealed that over 64 percent of patients who received Cartiva implants had device failure within the first week following the procedure.
In all the adverse events reported by the FDA concerning big toe implants from 2010 through 2018, 23.4 percent were linked with Cartiva implants. This fact was discovered by the study released by The Journal of Foot and Ankle Surgery.
In the early years following the introduction of Cartiva as an alternative treatment for arthrodesis, Cartiva implants elicited problems from people. Patients complained of more toe pain, a decrease in the size of the implant because of its shrinkage, and a fracture when the implant was buried into bone (subsidence).
Initially, scientists from the University Foot and Ankle Institute declared that Cartiva implants could provide long-term benefits and had no limitation in function. The Cartiva implant’s performance was predicted to be as over 90 percent of patients reported a significant reduction in discomfort, durability, and satisfaction.
Doctor. Bob Baravarian was one of the top researchers at the University Foot and Ankle Institute who was instrumental in developing the device.
After receiving complaints from patients of the implant’s failure, The surgeon notified the implant’s manufacturers of the problems to examine the problem. But, they were determined to reference the studies that substantiated the device’s safety. They didn’t pay attention to the concerns or take any steps to address these issues.
Dr. Bavarian found that the implants did not suffer damage in the revision surgeries. Dr. Bavarian speculated that Cartiva implant failures could be due to the defect in its design. The implant had an uncoated surface that could not hold onto the bone. It may have been kept in place if it had a slight irregularity to stop the slippage.
Before the implant, the implant is submerged in a water medium. After it is implanted, the implant must be maintained intact and hydrated to function effectively. Since the joint around the implant is impaired by arthritis, it becomes dehydrated. Because of this, the implant becomes smaller and caves in the bone.
Another thing to consider was that the bone surrounding could be weak to support the implant’s gel-like structure. It was unclear what amount of pressure would be applied to the Cartiva implant.
Since the negative effects began appearing, many surgeons have backed out from making recommendations or using Cartiva implants.
The concerns reported by those who were patients in the reports of adverse events for 144 plus included:
Cartiva implant failure symptoms could be severe pain and discomfort when walking and require revision procedures.
A joint replacement procedure after removing the implant that failed is not very beneficial in the long run for patients. A joint replacement requires the removal of a portion of the bone. This will cut down the length of the large toe, making it smaller than the other toes. A smaller toe can affect the balance of the body.
After joint replacement surgery, the large toe will stick up but not touch the ground, which could be painful.
Cathy Atkinson from Texas filed her Cartiva implant lawsuit in October 2022. The lawsuit claimed that the toe implant did not solve her pain. The plaintiff received her Cartiva toe implant in November 2018, and it didn’t improve her motion mobility. She had to undergo further painful revision surgeries to remove the device (December 2020) and re-evaluate the bone loss triggered by this implant (arthrodesis fusion procedure in 2021).
Attorney Gabriel Gesmer had endured two implant surgeries and was afflicted with nerve damage as well as loss of mobility and loss of bone as the result of the procedure. His suit claimed that manufacturers knew they were making ” unreasonably dangerous products due to non-compliance.”
In his lawsuit, he claimed the makers promoted a “defective and adulterated” device that caused his pain.
The plaintiff Sally Peterson received an implant for her toe in January 2018. The lawsuit claimed it was because the plaintiff “experienced the loss of mobility, nerve damage, and debilitating pain of the Left great toe, along with constant irritation and discomfort in the location of the artificial Cartiva device.”
She was treated for another implant removal procedure, additional treatments, and suffering and pain. The Cartiva implant suit was based on the fact the “defendants incorrectly stated in the updated label that 9.2% of Cartiva subjects and 12% of fusion subjects had the implant and hardware removed during the study.”
Lawyer Milberg Coleman Bryson Philips Grossman has filed a Cartiva implant case for the benefit of Plaintiff Gina Neil. Tammie Thompson brought a second lawsuit in March 2022. The lawsuit claimed that the failure of the Cartiva toe implant caused her suffering and pain. The details of the two suits are not yet available.
The Lawsuit Information Center mentions that approximately five Cartiva implant-related lawsuits were brought in 2022. Ms. Atkinson’s lawsuit was among them. The specifics of those lawsuits are:
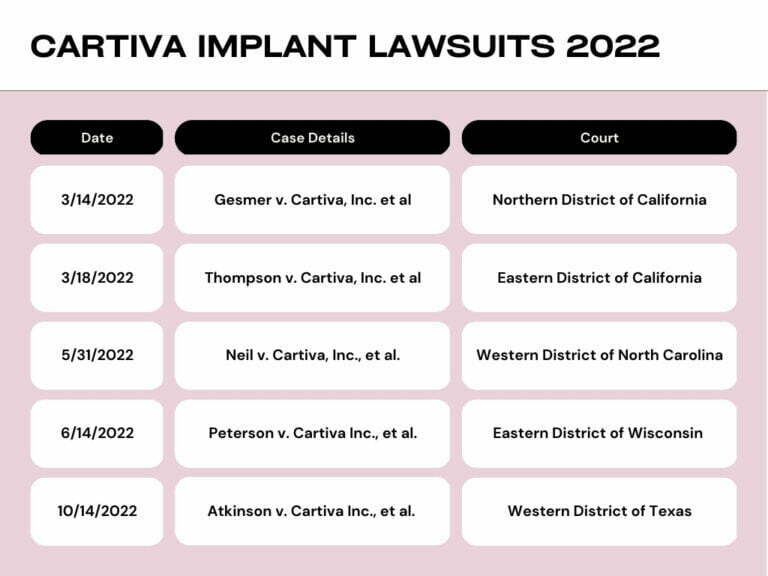
It is claimed that the cases of four others to one of Ms. Atkinson’s cases were dismissed in September 2022. Lawyers believe the four Cartiva implant lawsuits could have been settled out of the courtroom.
Several other people affected by Cartiva implants for the toe have begun making Cartiva implant lawsuits across the nation. If these lawsuits become overwhelming number and number, there are possibilities of consolidation into MDLs. There has yet to be a Cartiva class suit filed.
On the 12th of January 2023, the case of Cathy Atkinson’s Cartiva suit was dismissed voluntarily, with the possibility of reapplying this claim shortly. Experts believe that, like other claims dismissed earlier, this one could have been settled out of the court.
The Cartiva toe implant lawsuits brought against the manufacturer Cartiva Inc., Wright Medical Group, and Stryker included the following claims.
The manufactures
The lawsuit filed in California claimed that the defendants knew about the design defect from patients’ adverse event reports but withheld the details from doctors.
The criteria for pursuing the possibility of a Cartiva implant lawsuit comprise the following:
Contact an experienced Cartiva lawyer to handle your claim If you or your loved ones are affected by a Cartiva implant malfunction.
The medical documents should have been able to document the progress and diagnosis of your foot arthritis in specific detail. The date and details of your curative implant surgery and any issues, pain, the discomfort you experienced, and any procedures for revision and different therapies will all be important in determining if you get compensation.
A thorough examination of your records of medical documents can revive your claims and assist your Cartiva Implant case in coming to an effective conclusion.
LezDo TechMed has years of management experience in helping to defend product liability claims by providing fact-based medical records reviews. Upload your documents to them, and they will find your case’s strengths and weaknesses in the blink of an eye.
To finish,
Legal actions concerning Cartiva implants are currently in their early stages. Lawyers expect that Cartiva lawsuits may begin to grow in courts in the U.S. courts very soon.
All over the country, plenty of Cartiva Implant lawyers have taken on cases. To advance your claim, you should seek the guidance of an experienced attorney. Contact The Justice Now Experts today to Get Free Cartiva Implant Lawsuit Case Evaluation and you may be eligible for compensation.[/subscribe_to_unlock_form]
Need help?