Following that, the U.S. Food and Drug Administration (FDA) published millions of medical device injury reports last year that were previously hidden from the public; a growing number of lawsuits involving product liability are being brought against Bard in a claim that the company hid Bard Power port problems involving fractures and infections from patients and from the medical community.

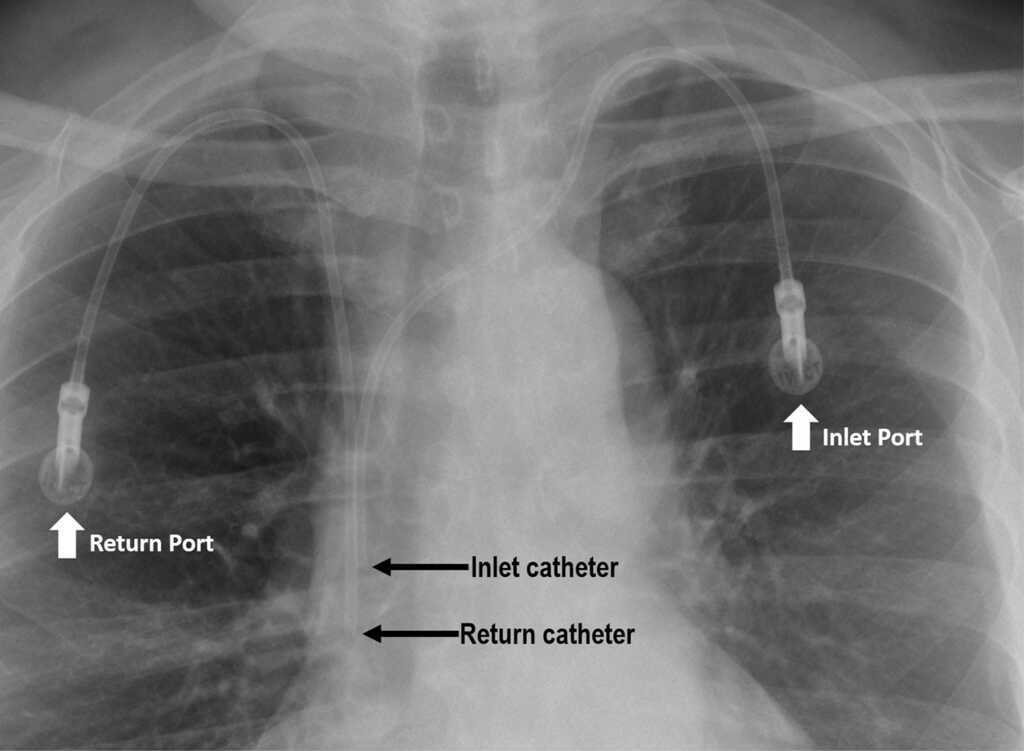
A Bard Power Port is a vessel access device that is inserted beneath the skin to create an accessible catheter port to facilitate the delivery of drugs to the bloodstream of a patient, for example, chemotherapy. It is composed of a primary injection port, through which the needle is placed to deliver medication, as well as a catheter made of a polyurethane tube that is used to provide the drug into the blood vessels.
Although the manufacturer has marketed the port catheters as secure and efficient, the newly released FDA adverse report has revealed an alarmingly high percentage of Bard Power Port problems over the last two decades that lawsuits are now alleging is due to a flawed design.
In this blog, TheJusticeNow.com will outline the allegations by Bard Power Port litigations and discuss how the company was able to hide information regarding fractures and migratory injuries for so long.
Lawsuits Allege Bard PowerPort Design Defects
The same claims have been made in lawsuits filed all over federal courts in the last several months, all of which indicate that issues in Bard Power Port and other compatible Groshong catheters have caused patients to suffer devastating and traumatic injuries.
In light of the common issues of fact and law that have been raised during the litigation escalating, A motion was filed in the last month to unify and condense the entirety of Bard Power Port lawsuits before a single judge for coordinated discovery and pretrial procedures.
The consequences suffered by each plaintiff differ and vary; all of the lawsuits assert the problems in Bard Power Port. Bard Power Port stems from an unsafe and defective design. This is a case of Chronflex components made from an unsuitable mix of barium sulfate and polyurethane.
The inclusion of barium sulfate within Chronoflex products has been shown to compromise the mechanical strength of the catheters made of polyurethane, per the lawsuits. This has led to a decrease in the power of the catheters, which can lead to micro-fractures, degradation cracks, fissuring, and cracks, which can cause pieces within the catheter to fall off and spread throughout the bloodstream or even build up bacteria.
Plaintiffs argue that Bard was aware or ought to have known about the design flaws. They also claim that the manufacturer deliberately kept details about Bard Power Port problems from consumers and the medical profession for many years.
How Bard Hid Power Port Problems For Decades
The FDA approved Bard to start selling PowerPort. PowerPort under the specific SS510(k) section of Medical Device Amendments to the Food, Drug, and Cosmetic Act. Contrary to the extensive and stringent FDA pre-market regulations for medical devices, approval under SS510(k) permits the commercialization of medical devices when the manufacturer can prove that the device is substantially comparable to devices that have been approved previously.
Manufacturers granted special authorizations through the SS510(k) provision could be exempted from the regular reporting requirements for adverse events. In the instance of Power Port, Bard was granted these reporting exemptions that required the company to file certain kinds of reports via the FDA’s Alternative Summary Reporting (ASR) program. This allowed the words about Bard Power Port issues to be hidden from healthcare professionals and the general public.
While Bard continued to report quarterly PowerPort problems through the ASR, which dates up to the early 2000s, The public, as well as the healthcare industry, needed to be given more warnings or details about the typical failures suffered by patients, as per the lawsuits, patients, as well as the medical profession, make a well-informed decision on whether or not to get the port catheter placed.
Problems Exposed by Reporting Guideline Changes
In May, a report that was an investigation by Kaiser Health News was published, which revealed how manufacturers were hiding issues and injuries to patients from the general public via ASR. ASR program, rather than sending reports of problems for the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database that is accessible to the public.
As a result of the public’s outrage in response, the FDA swiftly responded, announcing that it would release greater than the 6 million adverse reports of events that had never been made available to the general public. This included information on problems with Bard Power Port and various medical devices.
It was in February 2022 when the FDA finally released information on the adverse reactions, and it was later discovered that the information contained reports of problems related to Bard Power Port. Bard Power Port included incidents of pinch-off and device failures that the company classified as “known risks” or placement mistakes made by physicians.
Bard Blamed Doctors For Power Port Fractures & Migrations
Since Bard was not required to report certain kinds of adverse events in its unique classification, the lawsuits assert that Bard attempted to hide the flawed designs of the Power Port by classifying the issues that occurred under the heading of “known risks” or even more specifically, “compression or pinch-off events” that Bard tried to blame the implanting doctors.
Lawsuits now claim that Bard was aware that the Groshong and Power Port catheter design problems caused the pinch-off and fracture events, which caused fragments of catheter tubing to break off and move throughout the patient’s bodies.
Instead of addressing the risky design flaw, lawsuits assert that Bard tried to shift blame on the implanting doctors by releasing cautions that stated that when a doctor implants the device in a way that is not correct, it could result in a “compression or pinch-off” event.
The lawsuits claim that due to Bard’s failure to fix issues with the Bard Power Port, the affected individuals are suffering long-term effects, which have resulted in blood clots, cardiac arrhythmia and cardiac punctures, pulmonary embolisms, and tearing of blood vessels and organ damage. Several Bard Power Port lawsuits involving wrongful deaths are also being filed, claiming that the damage caused by this device caused fatal injuries.
Bard Power Port Catheter Infection Risks
The suit also claims that the design issues have led to severe infections in The Bard Power Port, indicating that the degradation in the barium sulphate pockets within the catheters results in a rougher surface for the catheter, which causes the accumulation and growth of microbes.
People are seeking Bard PowerPort lawsuits and claiming that the flawed design led to them developing deadly bloodstream infections or sepsis. In addition, they require replacement surgery and replacing ports that can be implanted.
Bard Didn’t Correct Known Problems With Power Ports
Although Bard has made a few statements that suggest that the complications suffered by patients who have Power Ports were the result of the implanting physician, the lawsuits show that the company knew, or ought to have realized that barium sulphate caused the degradation of the catheters made of polyurethane. However, they should have taken the necessary steps to correct the issue.
People are claiming that Bard did not adequately explore better alternatives to its design while claiming that the risks of infection and fracture could have been substantially diminished or avoided by structures that contain the radiopaque substance or by employing a different formulation of polymer.
However, instead of changing the design to make implantable port catheters more secure for users or launching the Bard Power Port recall, the manufacturer decided to keep with their marketing efforts to make the product safe, thus exposing more patients to the risk.
Have a Bard PowerPort Lawyer Review Your Case
As the litigation progresses, lawyers continue to provide free consultations and claims assessment to help those nationwide determine whether they could be qualified to receive a Bard PowerPort lawsuit settlement. There are still cases to be filed to address issues caused by the port for catheters, such as:
- Infections
- Blood Clots
- Perforations
- Catheter Fractures
- Wrongful Death
Submit information concerning your possible claim to be reviewed by an attorney. There aren’t any charges or costs unless an agreement or a recovery is negotiated.