Cartiva Toe Implant Lawsuit Information - Individuals who had issues with the big toe implant may be able to get settlement compensation through a Cartiva lawsuit. Learn more about the allegations that are being pursued as well as the criteria for potential Cartiva toe implant lawsuit awards.
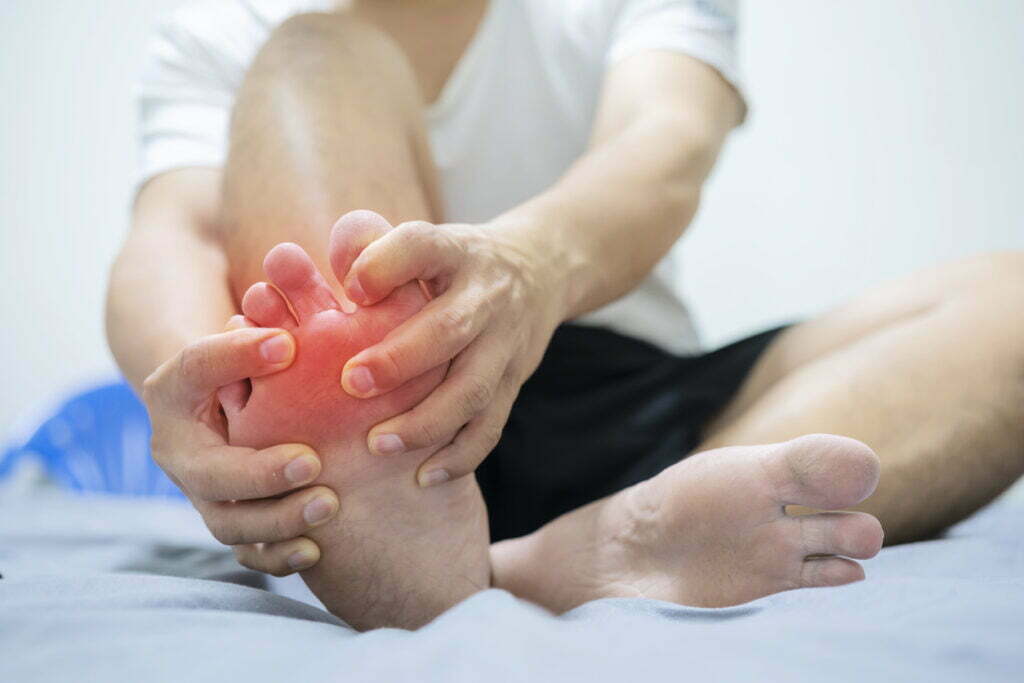
Overview of the Cartiva Lawsuit
Since 2016, the Cartiva synthetic cartilage implant has been touted as an alternative to big toe fusion surgery, with the maker hailing it as a groundbreaking toe implant meant to give pain relief for people with deteriorated cartilage in the big toe joint.
However, after only a few years, toe implant patients began reporting a high prevalence of Cartiva surgical complications, such as severe toe discomfort, loosening, fracture, and subsidence, which is where the implant slips into the bone.
As a result, people all around the country are filing Cartiva lawsuits against the manufacturer, saying that a flawed design causes the toe implant to fail and cause significant pain, frequently necessitating additional surgery and long-term incapacity.
Who May Be Eligible for a Cartiva Implant Lawsuit Payout?
Individuals who received the big toe implant and experienced any of the following issues may be eligible for financial compensation through a Cartiva lawsuit settlement:
- Implant Failure/Fracture
- Subsidence (implant sinks into the bone)
- Toe Fusion Surgery
- Replacement Surgery
- Revision Surgery
Cartiva implant lawyers analyse lawsuits on a contingency fee basis, which means that no costs or expenses are paid until there is a Cartiva settlement or lawsuit payment.
Cartiva Lawsuit Update for 2023
- January 2023 Update: A series of voluntary dismissals have been entered in early lawsuits filed, allowing plaintiffs to refile their claims in the future and raising speculation of confidential Cartiva toe implant settlements or an agreement to toll the statute of limitations.
- December 2022 Update: In the most recent of at least six Cartiva toe implant lawsuits filed this year, a Texas woman accused Stryker B.V., Wright Medical Group, N.V., and Cartiva, Inc. of negligently offering a defective medical device to consumers and failing to disclose known risks.
- November 2022 Update: A growing number of private orthopaedic practices have openly highlighted concerns about the high rate of Cartiva implant failure in patients in recent years. Due to the significant failure rate, many practices have essentially abandoned the use of Cartiva, opting for more successful alternative therapy alternatives.
Cartiva Implant Background and FDA Approval
Approximately 2.2 million people in the United States suffer from hallux limitus or hallux rigidus, which are forms of degenerative arthritis in the first metatarsophalangeal joint of the big toe. The disorders damage the cartilage in that joint, resulting in significant pain and limited range of motion in the big toe.
Prior to Cartiva, the sole choice for therapy was to remove the damaged big toe joint and fuse the toe bones together, a process known as big toe fusion or arthrodesis.
In 2015, the Cartiva Synthetic Cartilage Implant was released as an alternative to arthrodesis, including a cylindrical implant made of polyvinyl alcohol-based hydrogel (PVA) meant to offer gel-like cushioning to replace deteriorated cartilage in the big toe joint. The implant was marketed by the company as a novel alternative to big toe fusion surgery, and it was described as safe and successful.
The Cartiva implant was granted premarket approval by the US Food and Drug Administration in 2016, based on the findings of the “Motion” study. The Cartiva toe implant was stated to be significantly equivalent to big toe fusion surgery in treating individuals with damaged or degraded cartilage in the first metatarsophalangeal joint in the Motion study. The research also claimed there was only a 13.5% Cartiva implant failure rate.
Cartiva toe implant failure rates in real-world patient outcomes have been substantially higher, with patients experiencing worrisome rates of problems such as severe toe discomfort, loosening, fracturing, and subsidence, which is when the implant sinks into the bone.
What Causes Cartiva Implant Failure?
Several medical specialists have proposed explanations as to why the Cartiva implant fails. Among the most compelling arguments advanced for why the implant fails are:
- Improperly Textured – According to experts, the device’s round smooth surface on all surrounding sides may allow it to move. This can lead to implant loosening and joint failure.
- Cartiva Implant Shrinkage- Studies have shown that the water-based Cartiva implant may shrink after being implanted owing to dehydration. As a result, the implant may become loose and slip into the bone (subsidence).
- Experts have also speculated that the implant was not appropriately built to withstand the surrounding bone being weakened by arthritis, the ailment that Cartiva was supposed to treat. The lack of supporting bone, paired with an implant that is inadequately textured or shrinking, may cause the implant to loosen and slip into the bone.
Manufacturers Withheld Cartiva Implant Failure Data
Individuals have raised allegations against Cartiva Inc. Wright Medical Group, and Stryker in several Cartiva lawsuits filed throughout 2022, claiming the manufacturers withheld information about the increasing number of Cartiva implant failures for years in order to avoid a Cartiva recall and increase profits.
According to the lawsuits, each of the manufacturers that presently own Cartiva (Stryker) or formerly owned Cartiva since 2016 (Cartiva Inc. and Wright Medical Group) were aware of higher failure rates than the 13% stated to patients.
Plaintiffs contend that the manufacturer had sole knowledge of the possible Cartiva faults, citing more than 144 adverse event reports filed with the FDA involving loss of toe motion, discomfort, and high failure rates. Aside from the FDA findings, numerous studies have been published highlighting the rising risk of implant failures and consequences.
However, rather than completing their legal duty to report the increasing number of Cartiva implant failures and complications to the FDA and to notify the public, the medical device makers have failed to do so.
Cartiva Implant Complication Research
Several follow-up studies and case reports have never been able to replicate the Cartiva Motion clinical trial’s outcomes. In reality, evidence published in medical journals suggests that Cartiva implant failure rates may be up to 6 times greater than previously disclosed by the manufacturer.
November 2022: Cartiva Causes Increased Post Operative Pain Compared to Arthrodesis – Patients who received the Cartiva toe implant were more likely to suffer post-operative pain, according to preliminary data of an ongoing study published in the medical journal Foot & Ankle International.
The study looked at 100 patients who had Cartiva hemiarthroplasty, arthrodesis (joint fusing), or interpositional arthroplasty, and discovered that Cartiva patients had a worse overall combination of postoperative pain, function, and alignment than patients who had the other treatments.
October 2020: 79% of Cartiva Implants Failed Within Two Years – According to a study published by the American Orthopaedic Foot and Ankle Society, patients who receive Cartiva implants for the treatment of hallux rigidus face a 79% implant failure rate within two years.
The researchers discovered that 64% of individuals who received a Cartiva implant for hallux rigidus had device failure within four weeks of surgery. The study discovered that the probability of failure rose over time, with a 79% failure rate in patients 19 months after surgery.
Subsidence was the most commonly reported cause of Cartiva failure, in which the implant loosened and was able to sink into the toe bone, resulting in significant toe pain and, in some cases, revision surgery.
During the 19-month follow-up period, 43% of patients indicated that the Cartiva implant provided no effect in terms of alleviating toe discomfort or enhancing mobility.
October 2019: Nearly A Third of Cartiva Recipients Dissatisfied With Outcome – In another study published by the American Orthopaedic Foot and Ankle Society, Cedar Sinai Hospital researchers discovered that 30% of Cartiva SCI recipients were either unsatisfied or very dissatisfied with the procedure’s outcomes.
The study discovered that 50% of patients required a corticosteroid injection after receiving the implant, leading researchers to conclude that the Cartiva implant provided only minimal patient satisfaction, with a substantial proportion requiring additional treatment and workup post-operatively.
Examples of Cartiva Lawsuits
February 2023: Cartiva Toe Implant Medical Malpractice Lawsuit. Eugene Perez filed a complaint against the medical professionals who prescribed a Cartiva implant for his big toe pain caused by hallux rigidus. Perez argues that the medical practitioners violated their standard of care by proposing a Cartiva implant, which has a faulty design, is prone to failure, and is ineffective for treating his disease.
October 2022: Cartiva Lawsuit Over Failure Two Years After Toe Implant Surgery. Cathy Atkinson sued Cartiva manufacturers in the United States District Court for the Western District of Texas, saying she received a defectively manufactured Cartiva implant that failed, necessitating repeated procedures. In November 2018, Atkinson had a Cartiva implant, which failed only two years later, necessitating Cartiva removal surgery in December 2020 and arthrodesis fusion surgery in 2021.
Consult a Cartiva Lawyer About Your Case
If you or a loved one received a Cartiva toe implant and had complications that necessitated revision surgery, replacement surgery, or big toe fusion, submit information about your potential claim for review by a Cartiva experts to see if a settlement or lawsuit payout is available.
Our Experts offer no-cost claim analyses and consultations. There are no fees or charges unless your case is successful.

